Ethyl acetate
Are you searching for a solvent that combines efficiency, safety, and versatility? Look no further than ethyl acetate, a key ingredient in everything from decaffeinating tea and coffee to nail polish removers and beyond. Recognized for its excellent solvency properties, Ethyl acetate is the solution you need for a wide range of applications.
Versatile Applications: Whether you’re in the pharmaceutical, food and beverage, or cosmetic industry, ethyl acetate’s exceptional solvent properties make it indispensable. Its applications range from acting as a flavor enhancer in food to being a core ingredient in cleaning products, offering unparalleled versatility.
Safety and Compliance: Prioritizing safety, ethyl acetate is not only effective but also safe for use in food products and cosmetics, adhering to strict regulatory standards. Its low toxicity levels make it a safer choice than many chemical alternatives, ensuring the well-being of your employees and customers.
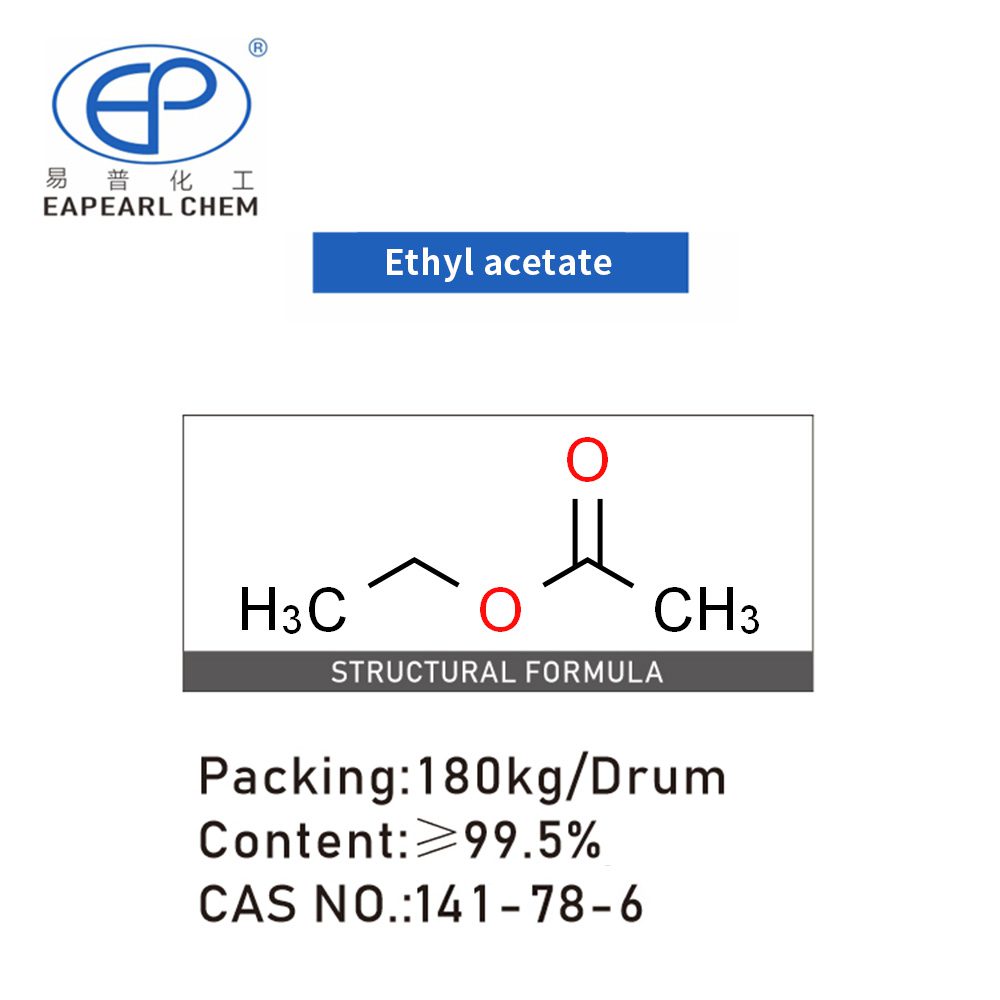

Ethyl Acetate, often abbreviated as ETAC, is a colorless liquid known for its sweet, fruity aroma reminiscent of pears. As a versatile organic compound, Ethyl Acetate belongs to the ester family, produced by the reaction between ethanol and acetic acid. This solvent’s popularity in various industries stems from its excellent solvent properties, low toxicity, and agreeable odor.
Synonyms: ETAC, acetic ester, Ethyl acetate, SPIRIT OF WINE, METHYLCARBINOL, REAGENT ALCOHOL, Acetic acid ethyl ester, METHYLATED SPIRIT INDUSTRIAL, METHYLATED SPIRIT MINERALISED
Nature: The nature of Ethyl Acetate is characterized by its volatile, colorless, and flammable properties. It has a boiling point of 77.1°C (170.78°F) and a distinctive sweet smell that makes it recognizable. Ethyl Acetate is soluble in most organic solvents but has limited solubility in water, making it an excellent solvent for organic synthesis, coatings, and extractions.
Table of Contents
Ethyl Acetate Packaging Information






| Ethyl acetate packaging | Capacity | 20GP | 40GP |
| drum | 180 kg /drum | total 80 drums, Net 14.4 ton | total 136 drums, Net 24.48 ton |
| IBC drum | 860 kg /IBC | total 20 IBC, Net 17.2 ton | total 30 IBC, Net 25.8 ton |
| ISO Tank | 20.5 ton /ISO Tank | 1 ISO Tank, Net 20.5 ton | N/A |
For ethyl acetate, we welcome you to test and check the quality, if you need a sample please contact our sales team to discuss your sample requirements, we believe that our product quality is suitable for the specific application. We provide samples free of charge but the shipping cost will be borne by you.
Applications of Ethyl Acetate
Ethyl acetate is a widely used solvent with a broad range of applications across various industries due to its excellent solvent properties, low toxicity, and agreeable odor. Here are detailed descriptions of its specific applications:
Food and Beverage Industry
Ethyl acetate is employed as a flavoring agent due to its sweet, fruity essence that enhances the aroma and taste of food products. It’s commonly used in:
- Confectionery: Enhancing flavors in candies and sweets.
- Beverages: Used in both alcoholic and non-alcoholic drinks to impart or enhance fruity flavors.
- Dairy Products: In ice cream and yogurt to simulate fruit flavors without using real fruits, thus reducing cost.




Pharmaceuticals
In the pharmaceutical industry, ethyl acetate serves multiple roles, notably:
- Solvent for Extractions: Used in the extraction and purification of antibiotics, certain vitamins, and other active pharmaceutical ingredients (APIs).
- Intermediate: In the synthesis of various drugs, ethyl acetate acts as a key solvent or reactant.
- Coating Agent: Employed in coating formulations for pills and tablets to ensure controlled release of the drug.
Cosmetics and Personal Care
Ethyl acetate’s mild nature and pleasant smell make it suitable for cosmetic applications, such as:
- Nail Polish Remover: A less aggressive alternative to acetone, it effectively dissolves nail polish without excessive drying of the skin and nails.
- Perfumes and Fragrances: Used as a solvent to dissolve fragrance compounds, contributing to the scent profile without overpowering the fragrance notes.
- Hair Care Products: In products like hairsprays, ethyl acetate acts as a solvent for resins and polymers, ensuring an even application.




Adhesives and Coatings
Its fast evaporation rate and solvency power make ethyl acetate ideal for use in:
- Paints and Varnishes: As a solvent in surface coatings, it helps in adjusting the viscosity and improving the application properties.
- Adhesives: Used in formulating glues for woodworking, fabrics, and crafts, where a quick set and strong bond are desired.
- Ink for Printing: In printing inks, it dissolves the resin, controls viscosity, and ensures a smooth print.
Laboratory Use
Ethyl acetate’s low toxicity and ease of removal make it a preferred solvent in laboratories:
- Organic Synthesis: Used as a reaction medium for various organic reactions due to its solvent properties.
- Chromatography: Employed as an eluent in column and thin-layer chromatography for the separation of mixtures.
- Extractions: Utilized in liquid-liquid extractions due to its ability to dissolve a wide range of organic compounds.




Decaffeination
Ethyl acetate is a natural, food-grade solvent used in the decaffeination process of coffee and tea. The process involves:
- Steaming: Coffee beans or tea leaves are steamed to open their pores.
- Extraction: The beans or leaves are then soaked in ethyl acetate, which selectively binds to the caffeine molecules.
- Evaporation: The ethyl acetate and caffeine are then removed through evaporation, leaving behind the decaffeinated product. This method is favored for its ability to efficiently remove caffeine while preserving the depth and integrity of the coffee or tea flavors.
Electronics and Cleaning
Ethyl acetate’s effectiveness as a solvent extends to the electronics industry and cleaning applications:
- Cleaning Solvent: It is used to clean circuit boards and electronic components because of its ability to dissolve residues without damaging the components.
- Precision Cleaning: In precision cleaning operations, ethyl acetate is valued for its fast evaporation rate, leaving behind no residue on optical devices, mechanical parts, and sensitive electronics.
- Degreasing: Its solvency power is employed in degreasing metals and plastics, preparing surfaces for further processing or finishing.




Agriculture
In the agricultural industry, ethyl acetate is utilized for its solvent properties in pesticide and herbicide formulations:
- Pesticide Production: It dissolves active ingredients for pest control solutions, ensuring they can be evenly spread and absorbed by plants.
- Herbicide Formulations: Ethyl acetate helps in creating effective weed control products by solubilizing the active compounds that target specific weeds without harming crops.
- Fungicides: In fungicide products, it acts as a carrier solvent, facilitating the application and effectiveness of the fungicide on plants. Its role in agriculture underscores the importance of solvent selection in achieving desired outcomes in pest, weed, and disease control.
Textile Industry
Ethyl acetate finds applications in the textile industry, particularly in:
- Dyeing and Printing: It solubilizes dyes for fabric dyeing and printing processes, ensuring even color distribution and penetration.
- Spot Cleaning: As a spot remover, it effectively dissolves adhesives, gum, and oil from fabrics without leaving stains.
- Finishing Processes: Used in textile finishing to dissolve coatings and treatments applied to fabrics, improving texture and appearance.




Synthetic Leather Production
In the production of synthetic leather, ethyl acetate is used as a solvent for:
- Polymer Solutions: It dissolves polymers that are then cast or coated onto fabric substrates to create synthetic leather.
- Plasticizers: Ethyl acetate helps in blending plasticizers into polymers, enhancing the flexibility and durability of synthetic leather products.
- Surface Treatments: It is involved in applying protective and aesthetic finishes to synthetic leather, contributing to its final texture and appearance.
The versatility of ethyl acetate as a solvent is evident from its widespread use in industries ranging from food and beverage to pharmaceuticals and cosmetics. Its selection is often based on its effectiveness, favorable safety profile, and minimal environmental impact, making it a solvent of choice for many applications. If you have any questions about the application of ethyl acetate, please contact our experts.
REQUEST A QUOTE FOR MORE DETAILS
Technical Data of Ethyl Acetate
| Name | Ethyl acetate |
| Synonyms | ETAC, acetic ester, Ethyl acetate, SPIRIT OF WINE, METHYLCARBINOL, REAGENT ALCOHOL, Acetic acid ethyl ester, METHYLATED SPIRIT INDUSTRIAL, METHYLATED SPIRIT MINERALISED |
| CAS | 141-78-6 |
| EINECS | 205-500-4 |
| Molecular Formula | C4H8O2 |
| Molar Mass | 88.1051 |
| Density | 0.898g/cm3 |
| Melting Point | -83.5℃ |
| Boling Point | 73.9°C at 760 mmHg |
| Flash Point | 26 °F |
| Water Solubility | 80 g/L (20℃) |
| Vapor Presure | 112mmHg at 25°C |
| Vapor Density | 3 (20 °C, vs air) |
| Appearance | Form: Liquid |
| Color: APHA: ≤10 | |
| pKa | 16-18(at 25℃) |
| LogP | 0.68-0.73 at 20-25℃ |
| Storage Condition | Store at +2°C to +25°C. |
| Stability | Stable. Incompatible with various plastics, strong oxidizing agents. Highly flammable. Vapour/air mixtures explosive. May be moisture sensitive. |
| Refractive Index | 1.373 |
| Physical and Chemical Properties | Colorless, flammable liquid with fruit flavor. |
| melting point -83.6 ℃ | |
| boiling point 77.1 ℃ | |
| relative density 0.9003 | |
| refractive index 1.3723 | |
| flash point 4 ℃ | |
| solubility, halogenated hydrocarbons, aromatic hydrocarbons and other organic solvents are miscible, slightly soluble in water. |
Ethyl Acetate Production
The production of ethyl acetate can be achieved through several methods, with the esterification of ethanol and acetic acid being the most common. Here is a detailed explanation of the specific production process, focusing on this prevalent method:
1. Raw Materials
The primary raw materials required for the production of ethyl acetate through esterification are:
- Ethanol (Ethyl Alcohol): A colorless, volatile liquid, also known as alcohol, derived from the fermentation of sugars or synthesized from ethylene.
- Acetic Acid: A colorless liquid with a distinctive sour taste and pungent smell, commonly used in vinegar besides its industrial applications.
2. Esterification Process
The esterification process is a chemical reaction between an alcohol (ethanol) and an acid (acetic acid) to form an ester (ethyl acetate) and water. The process involves several steps:
a. Mixing
- Ethanol and acetic acid are mixed in a reaction vessel. The typical molar ratio of ethanol to acetic acid used in the process ranges from 1:1 to 1:2.
b. Catalyst Addition
- A strong acid catalyst, usually sulfuric acid, is added to the mixture to accelerate the reaction. The catalyst facilitates the transfer of a proton from the acid to the alcohol, initiating the esterification process.
c. Heating and Reaction
- The mixture is heated to a temperature range of 60°C to 80°C (140°F to 176°F), which is sufficient to promote the esterification reaction without causing excessive evaporation of the reactants.
- Under these conditions, ethanol reacts with acetic acid to form ethyl acetate and water.
d. Removal of Water
- To drive the reaction towards the formation of more ethyl acetate (Le Chatelier’s principle), the water produced as a byproduct is continuously removed from the reaction mixture. This can be achieved through azeotropic distillation or by using a dehydration agent.
e. Purification
- Once the reaction is deemed complete, the mixture is distilled to separate ethyl acetate from the unreacted ethanol and acetic acid, as well as from the catalyst and any byproducts.
- The distillation process typically involves fractional distillation, where ethyl acetate, having a boiling point of 77.1°C (170.78°F), is vaporized and condensed, separating it from other components based on their boiling points.
f. Quality Control and Packaging
- The purified ethyl acetate is then subjected to quality control tests to ensure it meets the required specifications for purity, color, and odor.
- Upon passing quality control, the ethyl acetate is packaged in suitable containers for storage and distribution.
For ethyl acetate we can provide Reach certificate, details can be obtained by submitting an enquiry.
REQUEST A QUOTE FOR MORE DETAILS
How can we handle your order?


step 1
We will communicate with you within 24 hours after you send an enquiry.


step 2
If you need sample testing, we will send samples within 5 days,≤50kg, Express delivery recommended, usually called as DDU service; delivery time 5-7 days. Door to door service.


step 3
If you need bulk goods after the sample test is qualified, we will ship the goods to the port and keep the samples within 6 days after the order is confirmed. Sea shipping recommended, usually called as FOB, CFR, or CIF service.delivery time 10-45 days. Port to port service.


step 4
After waiting for you to receive the goods, we will arrange professional staff to pay a return visit within 7 days.
Ethyl Acetate: Storage and Environmental Disposal
Ethyl Acetate, a widely used solvent in various industries, requires careful handling, storage, and disposal to minimize risks to health, safety, and the environment.
Storage
Storage Conditions: Ethyl Acetate should be stored in a cool, well-ventilated area away from sources of ignition and heat, as it is highly flammable. The storage area should be equipped with explosion-proof ventilation and storage systems to mitigate risks. Containers of Ethyl Acetate should be tightly sealed to prevent leakage and minimize evaporation.
Storage Equipment: Storage containers and equipment must be made of materials compatible with Ethyl Acetate, such as stainless steel or certain types of plastic. The use of incompatible materials can lead to chemical reactions, compromising the integrity of the storage container and leading to leaks or spills.
Handling: Safe handling of Ethyl Acetate requires the use of personal protective equipment (PPE), including gloves, goggles, and respirators, to protect against inhalation and skin or eye contact. It is essential to ensure that work areas are well-ventilated and that spill containment measures are in place.
Environmental Disposal
Regulatory Framework: The disposal of Ethyl Acetate is regulated under environmental protection laws in most countries. These regulations mandate the proper treatment and disposal of Ethyl Acetate to prevent harm to the environment and public health.
Disposal Methods:
Ethyl Acetate should be disposed of as hazardous waste according to local environmental regulations. Preferred methods include:
- Incineration: Ethyl Acetate can be incinerated in a controlled environment at a facility equipped to handle chemical wastes, with measures in place to treat emissions.
- Chemical Treatment: Chemical treatment can neutralize Ethyl Acetate, transforming it into less harmful substances before disposal.
- Recycling: Whenever possible, Ethyl Acetate should be recycled or reclaimed for reuse, minimizing the amount of waste requiring disposal.
Safety Precautions: When storing or disposing of Ethyl Acetate, it is critical to follow safety guidelines to prevent accidents. This includes keeping the substance away from open flames, avoiding inhalation of fumes, and wearing appropriate PPE. In case of a spill, absorbents should be used to contain the liquid, and the area should be ventilated to disperse vapors.
Advantages of the Chinese Ethyl Acetate Market
The Chinese ethyl acetate market has emerged as a significant player on the global stage, driven by its robust manufacturing sector, strategic market positioning, and innovative approaches to production and distribution. This part explores the advantages of the Chinese ethyl acetate market, highlighting its impact on the global chemical industry.
Strategic Industrial Integration
China’s comprehensive industrial framework integrates ethyl acetate production with key consumer industries such as pharmaceuticals, coatings, and adhesives. This integration facilitates streamlined supply chains, reducing logistical challenges and enhancing the efficiency of production and distribution processes. The proximity of manufacturing plants to end-use industries minimizes transportation costs and time, contributing to a more sustainable and cost-effective market.
Scale of Production
The sheer scale of China’s chemical manufacturing sector, including ethyl acetate production, is unmatched. High-volume production capabilities enable economies of scale, making the Chinese market highly competitive in terms of pricing and supply reliability. This scale benefits global buyers by providing a stable supply of ethyl acetate, even in the face of fluctuating demand or supply chain disruptions in other parts of the world.
Technological Innovation
China’s commitment to technological advancement in chemical manufacturing has led to significant improvements in ethyl acetate production processes. Innovations in catalysis, process optimization, and waste reduction have not only enhanced production efficiency but also minimized environmental impact. These advancements ensure that Chinese ethyl acetate meets global quality standards while adhering to increasingly stringent environmental regulations.
Government Support and Regulatory Environment
The Chinese government’s support for the chemical industry, including ethyl acetate production, through policies, subsidies, and infrastructure development, has been a crucial factor in its growth. Additionally, regulatory frameworks aimed at improving environmental performance and promoting high-tech industries have spurred innovation and investment in cleaner, more efficient production technologies.
Global Trade Dynamics
China’s strategic focus on export markets has positioned it as a key supplier of ethyl acetate globally. With well-established trade relations and logistics networks, Chinese producers can efficiently serve international markets, ensuring a consistent and reliable flow of ethyl acetate to meet global demand. The country’s participation in international trade agreements further facilitates access to key markets, reinforcing its position as a global supplier.
Our Team
FAQs of Ethyl Acetate
A1: Ethyl Acetate is a versatile organic solvent widely used across industries such as pharmaceuticals, cosmetics, food and beverage for flavoring, and in the manufacturing of paints, coatings, and adhesives. Its excellent solvency properties make it ideal for a variety of applications.
A2: Yes, Ethyl Acetate is considered safe for use in food products. It is commonly used as a flavor enhancer in foods and beverages, subject to regulatory approvals such as those from the FDA or corresponding international bodies, which ensure its safety and compliance with food-grade standards.
A3: Ethyl Acetate should be stored in a cool, well-ventilated area away from heat sources and open flames, as it is highly flammable. Proper handling includes wearing appropriate personal protective equipment (PPE), such as gloves and goggles, to prevent skin and eye contact. Ensure good ventilation in the working area to avoid inhalation of vapors.
A4: Ethyl Acetate is considered to have a low toxicity level to humans and the environment. It is biodegradable and less harmful compared to other solvents. However, responsible handling and disposal practices should be followed to minimize any potential environmental impact.
A5: Purchasing Ethyl Acetate requires confirmation of the buyer’s qualifications for handling and storing dangerous chemicals. This typically involves:
- A valid business license indicating authorization to purchase and use chemical solvents.
- Compliance with local and national regulations on the storage and handling of flammable substances.
- Adequate training and facilities for safe handling and storage, as per Occupational Safety and Health Administration (OSHA) guidelines or equivalent standards.
A6: Yes, Ethyl Acetate is often considered safer and less aggressive than acetone when used in nail polish removers. It is less drying to the skin and nails and offers a more pleasant scent, making it a preferred choice for many cosmetic products.
A7: Absolutely. We offer 100g-200g samples, with the client only covering shipping costs.
A8: Standard lead times are approximately 2-4 weeks, varying based on order size and destination.
A9: Our standard payment terms include a 30% advance and the balance against delivery, but terms can be negotiated for long-term partnerships.
A10: Yes, we offer comprehensive after-sales support, addressing any post-purchase queries or concerns.
A11: As a supplier, in order to provide you with an accurate quote for your product, please inform us of the quantity you require, the required purity specifications, any specific packaging needs, your shipping location, and whether your application requires any customization requirements or certifications.


