Methyl acetate
Discover the power of high-quality Methyl Acetate, the versatile solvent that’s transforming industries worldwide. From pharmaceuticals to paints and adhesives, our premium Methyl Acetate offers unmatched purity and performance for your specific applications. Embrace a solution that combines efficiency, safety, and environmental responsibility.
Methyl Acetate, known for its excellent solvent properties, plays a pivotal role in manufacturing processes across various sectors. Its fast evaporation rate and pleasant aroma make it a preferred choice for formulations requiring quick drying and minimal residue. By choosing our Methyl Acetate, you’re opting for a product that ensures consistent quality and reliability for your operations.
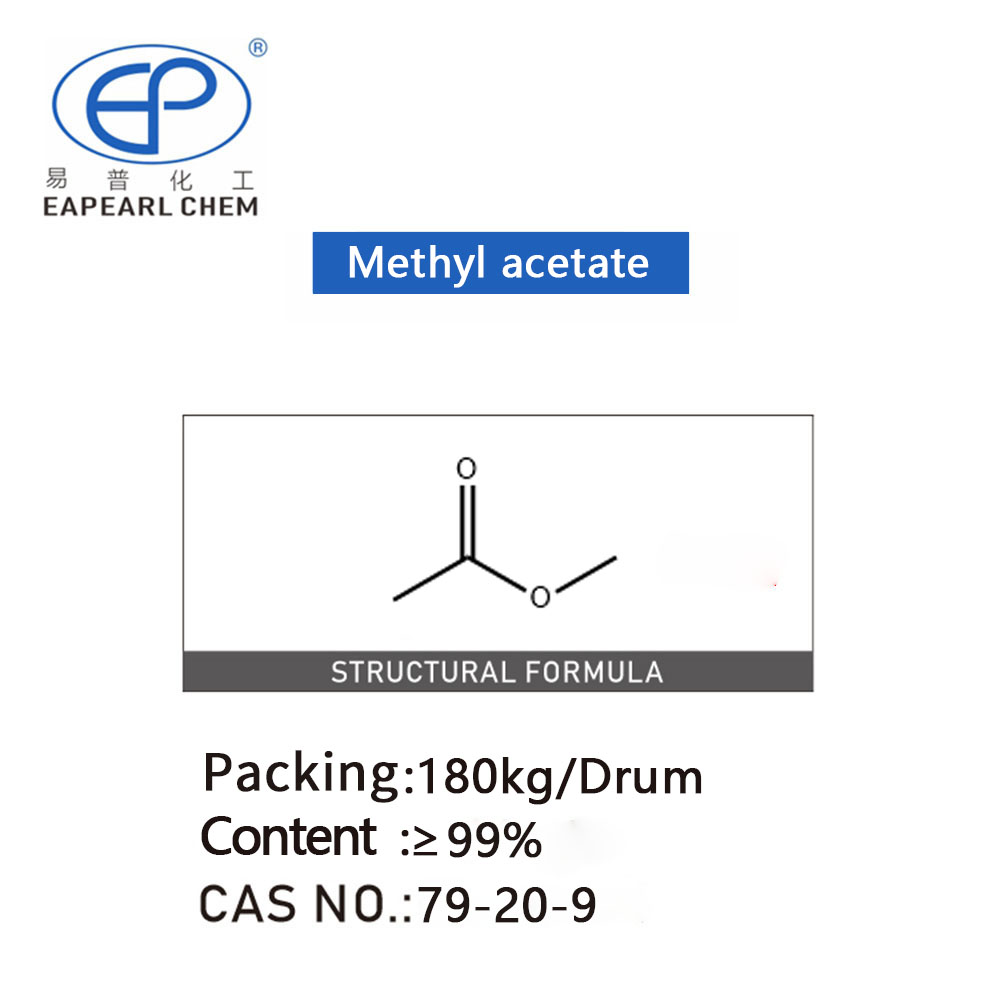

Introduction:Methyl Acetate, a solvent known for its wide array of applications and distinctive properties, stands as a pivotal chemical in various industrial sectors. This organic compound, represented by the chemical formula CH3COOCH3, merges versatility with efficiency, making it an essential ingredient in numerous manufacturing processes.
Synonyms:Metile, methylethanoate, methyle(acetatede), Acetate de methyle, Acetic acid methyl ester, methylesterkiselinyoctove, methyle(acetatede)(french);
Nature and Properties: Characterized by its clear, colorless appearance and pleasant odor, Methyl Acetate is a volatile, flammable liquid at room temperature. Boasting a boiling point of 56.9°C (134.4°F) and a melting point of -98°C (-144.4°F), it exemplifies a substance designed for a broad spectrum of applications due to its favorable physical and chemical properties. Its solubility characteristics, particularly its miscibility with organic solvents and limited water solubility, further underscore its utility in industrial applications.
Table of Contents
Methyl Acetate Packaging Information






| Methyl acetate packaging | Capacity | 20GP | 40GP |
| drum | 180 kg /drum | total 80 drums, Net 14.4 ton | total 136 drums, Net 24.48 ton |
| IBC drum | 800 kg /IBC | total 20 IBC, Net 16 ton | total 32 IBC, Net 25.6 ton |
| ISO Tank | 18.5 ton /ISO Tank | 1 ISO Tank, Net 18.5 ton | N/A |
For methyl acetate, we welcome you to test and check the quality, if you need a sample please contact our sales team to discuss your sample requirements, we believe that our product quality is suitable for the specific application. We provide samples free of charge but the shipping cost will be borne by you.
Applications of Methyl Acetate
Methyl acetate is a versatile solvent with a wide range of applications across various industries. Its properties, such as a pleasant odor, low boiling point, and excellent solvency, make it an ideal choice for numerous specific uses. Here, we delve into the detailed applications of methyl acetate:
Paints and Coatings
Methyl acetate is integral to the formulation of both indoor and outdoor paints and coatings. Its rapid evaporation rate facilitates faster drying times, which is crucial for applications requiring quick turnaround, such as in automotive and construction painting jobs. Moreover, methyl acetate helps in reducing paint viscosity without compromising the paint’s opacity or color properties, allowing for easier application and smoother finishes. Its use in eco-friendly paint formulations is increasingly important, as it helps manufacturers meet stricter VOC regulations without sacrificing performance.



Adhesives and Sealants
In the production of adhesives and sealants, methyl acetate acts not only as a solvent but also as a carrier for the adhesive components, ensuring uniform distribution and penetration for optimal bonding. It is particularly effective in the manufacture of hot-melt adhesives, pressure-sensitive tapes, and sealants used in packaging, electronics, and laminates. Its ability to dissolve a wide range of polymers and resins makes it versatile for various formulations, contributing to the strength and durability of the adhesive bonds.
Pharmaceuticals
The pharmaceutical industry benefits from methyl acetate’s high purity and solvent efficiency, especially in the extraction and refinement of delicate compounds such as antibiotics and hormones. Its controlled evaporation rate and compatibility with other solvents make it ideal for precision extraction processes, where maintaining the integrity and potency of the pharmaceutical compounds is paramount. Methyl acetate is also used in the crystallization of APIs, ensuring the production of pure, effective pharmaceutical products.




Cosmetics and Personal Care Products
Methyl acetate’s role extends to the formulation of fast-drying nail polish removers and perfumes, where its pleasant smell and low toxicity are particularly valued. It efficiently dissolves nail polish, allowing for easy removal, while in perfumes, it acts as a carrier for fragrances, enhancing the diffusion of scents. Its application in hair care products, such as hair sprays and gels, is also notable for providing a quick-drying effect without leaving residues, ensuring a clean, polished finish.
Food Industry
In food processing, methyl acetate’s solvent properties facilitate the efficient extraction of flavors, fragrances, and essential oils, which are critical in the production of food additives, flavorings, and concentrates. Its use in decaffeination processes for coffee and tea highlights its effectiveness in selectively extracting caffeine while preserving the taste and aroma of the final product. The solvent’s volatility ensures that it is fully removed from the food products after processing, maintaining the quality and safety of the food items.




Chemical Synthesis
Beyond its role as an intermediate, methyl acetate is pivotal in the synthesis of vinyl acetate, an essential monomer for producing polyvinyl acetate (PVA) and polyvinyl alcohol (PVOH), which are used in adhesives, paints, and textile sizing. Its involvement in carbonylation reactions to produce acetic anhydride, another valuable chemical intermediate, showcases its versatility in chemical manufacturing. Methyl acetate’s application in the production of high-purity solvents and reagents for analytical and research purposes further demonstrates its broad utility in the chemical industry.
These detailed applications of methyl acetate underscore its critical role in diverse industrial processes, from manufacturing and pharmaceuticals to cosmetics and food production. Its versatility, combined with favorable solvent properties, makes it an invaluable component in the formulation and production of a wide array of products.
REQUEST A QUOTE FOR MORE DETAILS
Technical Data of Methyl Acetate
| Name | Methyl acetate |
| Synonyms | Metile, methylethanoate, methyle(acetatede), Acetate de methyle, Acetic acid methyl ester, methylesterkiselinyoctove, methyle(acetatede)(french); |
| CAS | 79-20-9 |
| EINECS | 201-185-2 |
| Molecular Formula | C3H6O2 |
| Molar Mass | 74.08 |
| Density | 0.934 g/mL at 25 °C |
| Melting Point | -98 °C (lit.) |
| Boling Point | 57-58 °C (lit.) |
| Flash Point | 3.2°F |
| JECFA Number | 125 |
| Water Solubility | 250 g/L (20 ºC) |
| Solubility | 250g/l |
| Vapor Presure | 165 mm Hg ( 20 °C) |
| Vapor Density | 2.55 (vs air) |
| Appearance | Solution |
| Color | Clear colorless to slightly pale yellow |
| Odor | Slightly acrid, sweet; fragrant. |
| Exposure Limit | TLV-TWA 200 ppm (~610 mg/m3) (ACGIH,MSHA, and OSHA); TLV-STEL 250 ppm(~760 mg/m3) (ACGIH); IDLH 10,000 ppm(NIOSH). |
| Maximum wavelength(λmax) | [‘λ: 255 nm Amax: 1.0’, , ‘λ: 275 nm Amax: 0.1’,, ‘λ: 300 nm Amax: 0.01’] |
| Merck | 146,008 |
| BRN | 1736662 |
| Storage Condition | no restrictions. |
| Stability | Stable. Extremely flammable – readily forms explosive mixtures with air. Note low flash point and wide explosion limits. Incompatible with strong oxidizing agents, strong bases, strong acids, nitrates |
| Explosive Limit | 3.1-16%(V) |
| Refractive Index | n20/D 1.361(lit.) |
| Physical and Chemical Properties | Characteristics of colorless liquid, with aromatic flavor. |
| melting point -98.1 ℃ | |
| boiling point 57 ℃ | |
| relative density 0.4330 | |
| refractive index 1.3594 | |
| flash point -10 ℃ | |
| solubility, the ethers are miscible, and the solubility in water is 31.9g/100ml(20 °c). | |
| HS Code | 2915 39 00 |
| Toxicity | LD50 orally in Rabbit: > 5000 mg/kg LD50 dermal Rat > 2000 mg/kg |
| elative polarity | 0.253 |
| olfactory Threshold | 1.7ppm |
| LogP | 0.18 at 20℃ |
Methyl Acetate Production
The production of Methyl Acetate can be achieved through several methods, with the two most common being the esterification of acetic acid with methanol and the carbonylation of methanol. Here’s a detailed look into these specific production processes:
1. Esterification of Acetic Acid with Methanol (Acid-Catalyzed Esterification)
This method is the most traditional and widely used approach for producing Methyl Acetate. It involves reacting acetic acid (CH3COOH) with methanol (CH3OH) in the presence of a strong acid catalyst.
Process Steps:
- Reactant Preparation: Acetic acid and methanol are prepared and purified to remove impurities that could inhibit the reaction or poison the catalyst.
- Catalyst Addition: A strong acid catalyst, typically sulfuric acid (H2SO4) or hydrochloric acid (HCl), is added to the reaction mixture to accelerate the esterification process.
- Reaction: The mixture is heated under controlled conditions in a reactor. The esterification reaction follows the equation: CH3COOH+CH3OH→CH3COOCH3+H2O
- Product Recovery: The reaction mixture is then subjected to distillation to separate the Methyl Acetate from the water, unreacted acetic acid, methanol, and the catalyst.
- Purification: Further purification steps, such as fractional distillation, may be required to achieve high-purity Methyl Acetate.
- Recycling: Unreacted acetic acid and methanol can be recycled back into the reactor for improved efficiency and reduced waste.
2. Carbonylation of Methanol (Methanol Carbonylation)
An alternative method for producing Methyl Acetate is through the carbonylation of methanol, a process that directly introduces carbon monoxide into methanol to form Methyl Acetate and water.
Process Steps:
- Reactant Preparation: Methanol is purified, and carbon monoxide is sourced, often as a byproduct from other industrial processes.
- Catalyst Addition: A catalyst, commonly a rhodium or iridium complex, is introduced to facilitate the carbonylation reaction at lower temperatures and pressures compared to the traditional esterification process.
- Reaction: The reaction is carried out under pressure in a specially designed reactor, where methanol reacts with carbon monoxide to produce Methyl Acetate and water, following the equation: CH3OH+CO→CH3COOCH3+H2O
- Product Recovery and Purification: Similar to the esterification process, the product mixture is distilled to separate Methyl Acetate from water and any unreacted methanol. Further purification steps ensure the production of high-purity Methyl Acetate.
- Recycling: The process is designed to recycle unreacted methanol and carbon monoxide back into the reactor, enhancing the overall efficiency and sustainability of the production process.
Both methods have their advantages and are chosen based on economic, environmental, and technical considerations. The esterification process is well-established and straightforward, making it suitable for facilities that already produce or have easy access to acetic acid and methanol. The carbonylation method, on the other hand, is highly efficient and can be more cost-effective at large scales, especially with advancements in catalyst technology that minimize side reactions and enhance the selectivity towards Methyl Acetate.
REQUEST A QUOTE FOR MORE DETAILS
How can we handle your order?


step 1
We will communicate with you within 24 hours after you send an enquiry.


step 2
If you need sample testing, we will send samples within 5 days,≤50kg, Express delivery recommended, usually called as DDU service; delivery time 5-7 days. Door to door service.


step 3
If you need bulk goods after the sample test is qualified, we will ship the goods to the port and keep the samples within 6 days after the order is confirmed. Sea shipping recommended, usually called as FOB, CFR, or CIF service.delivery time 10-45 days. Port to port service.


step 4
After waiting for you to receive the goods, we will arrange professional staff to pay a return visit within 7 days.
Methyl Acetate: Storage and Environmental Disposal
Storage of Methyl Acetate
Methyl acetate should be stored in a cool, well-ventilated area away from sources of ignition and heat. Storage containers should be tightly sealed to prevent leakage and evaporation. The storage area must be equipped with explosion-proof electrical equipment and fittings due to the compound’s flammability. Grounding and bonding procedures should be implemented to prevent static discharge. Secondary containment measures are recommended to manage leaks or spills effectively.
Container Material
Suitable storage containers include those made of stainless steel, carbon steel, and certain plastics such as polyethylene, which are resistant to methyl acetate’s solvent properties. Containers should be clearly labeled with the chemical’s identity, hazard classification, and handling instructions.
Fire Prevention
Considering methyl acetate’s high volatility and flammability, storage facilities must be equipped with appropriate fire-fighting measures, including foam extinguishers, dry chemical extinguishers, and sprinkler systems. A comprehensive fire safety plan should be in place, incorporating emergency procedures and regular safety drills.
Environmental Disposal of Methyl Acetate
Disposal Considerations
The disposal of methyl acetate must be conducted in a manner that minimizes environmental impact. Improper disposal can lead to soil and water contamination, adversely affecting aquatic life and potentially entering the human food chain. Disposal practices must comply with local, national, and international environmental regulations.
Incineration
Controlled incineration in facilities equipped with scrubbers and filters to remove harmful emissions is the preferred method for disposing of large quantities of methyl acetate. This process reduces the compound to water and carbon dioxide, significantly mitigating its environmental impact.
Waste Treatment Facilities
For smaller quantities or mixed chemical waste containing methyl acetate, utilization of certified hazardous waste treatment facilities is recommended. These facilities have the capability to handle and process chemical waste safely, ensuring that disposal practices meet environmental standards.
Recycling and Recovery
Whenever possible, recycling or recovery of methyl acetate should be considered. Solvent recovery systems can condense and reclaim methyl acetate from industrial processes for reuse, reducing the need for disposal and the consumption of new materials.
Advantages of the Chinese Methyl Acetate Market
The Chinese methyl acetate market has experienced significant growth over the past few decades, becoming a pivotal player in the global chemical industry landscape. This growth is attributed to several key advantages that China offers in the production and distribution of methyl acetate, a solvent widely used in paints, adhesives, pharmaceuticals, and many other industrial applications. This part explores the various advantages of the Chinese methyl acetate market, including its vast manufacturing base, strategic global positioning, government policies, and innovation capabilities.
Extensive Manufacturing Infrastructure
One of the primary advantages of the Chinese methyl acetate market is its extensive and sophisticated manufacturing infrastructure. China hosts some of the world’s largest chemical production facilities, equipped with state-of-the-art technology and optimized for high efficiency and low-cost operations. This extensive infrastructure enables China to produce methyl acetate at a scale and cost that few other countries can match, making it a leading supplier in the global market.
Strategic Global Positioning
China’s strategic positioning as a central hub in the global supply chain significantly enhances its methyl acetate market. With access to major shipping routes and a vast logistics network, including ports, railways, and highways, China efficiently exports methyl acetate to international markets. This connectivity reduces transportation costs and delivery times, making Chinese methyl acetate more competitive on the global stage.
Supportive Government Policies
The Chinese government has historically prioritized the development of the chemical industry, including the methyl acetate sector. Supportive policies, including subsidies, tax incentives, and research and development grants, have bolstered the growth and competitiveness of Chinese methyl acetate manufacturers. Furthermore, the government’s focus on environmental standards has led to the adoption of cleaner and more sustainable production methods, improving the global perception of Chinese chemical products.
Innovation and Research & Development (R&D)
Innovation is another cornerstone of the Chinese methyl acetate market. Chinese companies and research institutions invest heavily in R&D to improve production processes, reduce costs, and develop new applications for methyl acetate. This commitment to innovation not only enhances the efficiency and sustainability of methyl acetate production but also positions Chinese manufacturers as leaders in the field, capable of meeting diverse and evolving market demands.
Access to Raw Materials
China benefits from domestic access to key raw materials needed for methyl acetate production, such as methanol and acetic acid. The availability of these raw materials within the country reduces dependency on imports, lowers production costs, and ensures a stable supply chain. This advantage is critical for maintaining uninterrupted production and meeting both domestic and international demand.
Expanding Domestic Market
The domestic demand for methyl acetate in China is another significant advantage. With rapid industrialization and growth in sectors such as automotive, pharmaceuticals, and electronics, the demand for methyl acetate within China has surged. This expanding domestic market provides a strong foundation for Chinese manufacturers, allowing them to scale up production and innovate while serving global customers.
Our Team
FAQs of Methyl Acetate
A1: Methyl Acetate is a versatile solvent with applications across various industries. It is commonly used in the production of paints, coatings, adhesives, pharmaceuticals, and cosmetics. Its quick evaporation rate and solvent properties make it ideal for formulations where a fast-drying solvent is required.
A2: Qualifications include:
- A valid business license indicating operation within relevant industries.
- Compliance with the Occupational Safety and Health Administration (OSHA) guidelines or equivalent safety standards in your jurisdiction.
- Specific certifications or permits for handling hazardous materials, depending on local and national regulations.
- A documented safety plan detailing storage, handling, and disposal procedures for hazardous chemicals.
A3: Safety considerations include:
- Using appropriate personal protective equipment (PPE), such as gloves and goggles.
- Ensuring good ventilation in areas where Methyl Acetate is used or stored.
- Preventing exposure to open flames or high heat sources due to its flammability.
- Adhering to storage guidelines to prevent leaks and minimize evaporation.
A4: Methyl Acetate should be stored in a cool, well-ventilated area away from direct sunlight and heat sources. Use containers made of materials compatible with Methyl Acetate, such as stainless steel or certain plastics. Ensure containers are tightly sealed and clearly labeled. Implement spill containment measures in storage areas.
A5: Dispose of Methyl Acetate through certified hazardous waste disposal services. Avoid releasing into the environment, as it can affect water sources and wildlife. Follow local regulations for hazardous waste disposal, and consider recycling or recovery options where available to minimize environmental impact.
A6: Methyl acetate offers fast evaporation, low toxicity, and excellent solvency for coatings, adhesives, and inks. It’s cost-effective and widely used in pharmaceuticals and cosmetics, providing a balance of performance and safety in industrial and consumer products.
A7: Absolutely. We offer 100g-200g samples, with the client only covering shipping costs.
A8: Standard lead times are approximately 2-4 weeks, varying based on order size and destination.
A9: Our standard payment terms include a 30% advance and the balance against delivery, but terms can be negotiated for long-term partnerships.
A10: Yes, we offer comprehensive after-sales support, addressing any post-purchase queries or concerns.
A11: As a supplier, in order to provide you with an accurate quote for your product, please inform us of the quantity you require, the required purity specifications, any specific packaging needs, your shipping location, and whether your application requires any customization requirements or certifications.


